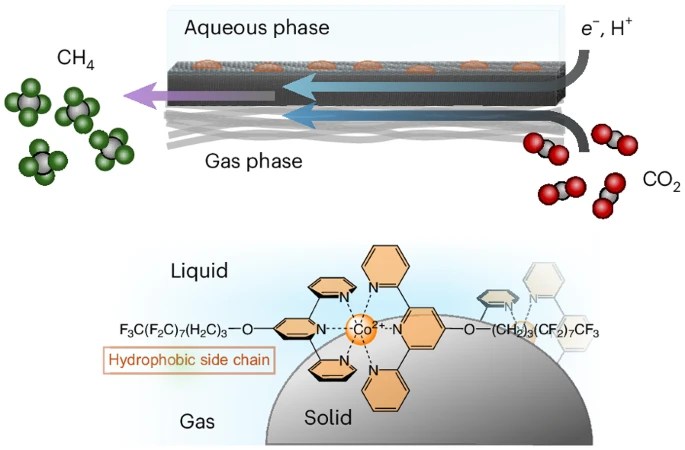
After a long review process, our joint paper with the Kornienko Lab in Bonn was finally published in Nature Chemistry (read paper here). The first experiments were performed by Max Kutter back in 2018! The paper shows how a mediocre homogeneous catalyst was turned into a highly active hetrogeneous electrocatalyst for CO2 reduction through the use of fluorine chemistry. Making a molecular cobalt terpyridine catalyst highly hydrophobic enables it to self-assemble on the surface of a gas-diffusion electrode, which alters its product selectivity from CO formation to the selective formation of CH4 at high current densities. We show that in this environment, proton transfer occurs via terpyridine ligands serving as a proton conduit. Free access to the paper is available here.
